Veeva Vault can drastically streamline your organization’s operations as long as it meets administrators’ expectations. Updating your Vault configuration ensures your system is optimized and fits your needs. Here are 5 strategies for making that happen.
Life sciences organizations face data migration issues and non-compliance when using multiple applications for content management. Veeva Vault is a cloud-based content management system that integrates various applications to address these issues. While Veeva simplifies data organization, system optimization is still necessary.
Optimizing Veeva software involves refining system configurations, settings, and workflows to maximize efficiency and compliance. Optimization ensures the software aligns with the organization’s operational needs, keeping content accessible and up to date throughout the product lifecycle.
One essential application in the Vault suite is Regulatory Information Management (Vault RIM). It manages product information, submission documents, and dossiers seamlessly, providing comprehensive support for compliance and efficient data management. Veeva Vault RIM is comprised of the following components:
- Registrations: Managing product registrations and health authority interactions.
- Submissions: Creating and managing submission content as well as submission planning and tracking.
- Submission Publishing: Building compliant submissions and submitting them to health authorities via an electronic gateway.
- Submissions Archive: Storing and viewing the complete history of the regulatory submission.
Housing regulatory processes on a single, integrated platform such as Vault RIM improves organizations’ visibility, data quality, and agility. What’s most important, however, is the display setup of these resources, as it ensures users can easily interpret and access vital information needed for decision-making.
What Is Veeva Vault Configuration?
Veeva Vault configuration is the back-end development that produces an application’s interface. Think of Veeva Vault’s various applications as puzzle pieces, and the configuration is your organization’s placement of those pieces in a strategic manner.
When efficiently assembled, the pieces create an interactive and positive user experience. When pieces are ineffectively organized, inaccurate data entry, submission delays, and security breaches may arise. Overall, misconfiguration could undermine the organization’s ability to manage regulatory information effectively, posing a non-compliance risk.
Be sure to utilize Vault RIM to its full extent to mitigate inconsistencies. For instance, there are various filters, fields, and reports that can populate a page. Applying the appropriate fields to a page improves workability:
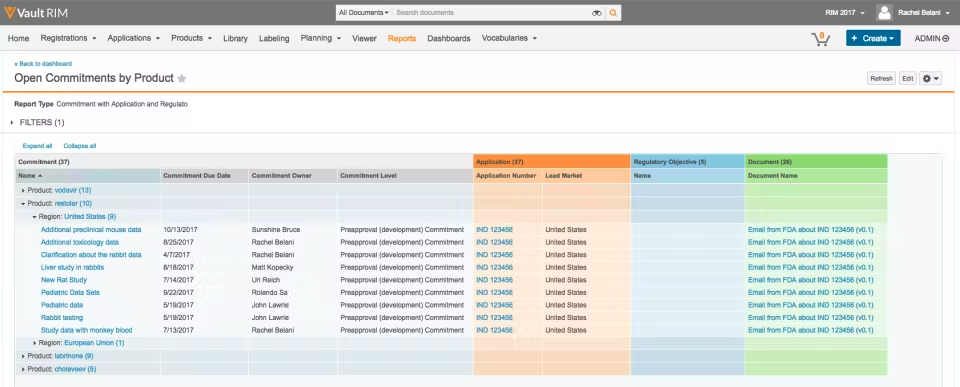
These components are entirely customizable. Items such as available fields, field values, and object identifiers can all be adjusted to reflect specified information to product managers.
Vault configuration and its ongoing review ensure:
Compliance
Life sciences regulations are stringent but subject to change. Optimizing your configuration verifies all documentation and data complies with the latest guidelines. Staying up to date reduces the risk of non-compliance, which results in delays, fines, and other penalties.
Data Integrity
Well-maintained data effectively communicates product performance. Optimizing your configuration certifies the accuracy and consistency of data across all departments and all stages of a product’s life cycle.
User Adoption
User adoption is the degree to which employees embrace and effectively utilize new systems. By configuring your systems, you tailor Vault applications to fit your employees’ specific needs. Employee satisfaction helps smooth the transitions of data migration and operational changes.
5 Tips for Optimizing Your Veeva Vault Configuration
Configuration improves interoperability and communication across departments through system unification. By taking these five steps, you can optimize your Veeva Vault configuration, promoting an improved product workflow.
1. Understand Regulatory Requirements
Compliance in the life sciences industry is non-negotiable, and its fast-paced nature can have a punitive impact on organizations that can’t keep up. Navigating the intricate web of rules and standards is essential to ensure product safety and quality.
Compliance software provides a global, authoritative source for regulatory documents and information. Content and data converge on a single platform to unify registrations, submissions, publication, and an archived history of submitted publications. When compliance information is accessible in a centralized location, the organization gains a deep understanding of relevant regulatory requirements.
How does configuration optimize your system?
Adopting a single, connected application promotes collaboration on the latest regulatory version. In doing so, review times are shortened, and a compliant product is brought to the market sooner.
2. Right-Size Your Security Model
Your organization’s security model should be simple and easy to scale. Finer access control means that security measures can adapt to your organization’s changing needs over time.
How does this optimize your system?
A personalized security model guarantees that unauthorized users cannot intercept or access data they are not supposed to, simplifying role responsibilities and employee satisfaction.
License types and security profiles, for example, assign permission sets to individual users. These configurations can be tailored to each user’s needs, or permissions can be assigned to broader roles or groups within the organization.
3. Leverage Analytics & Reporting
Reporting tools enable your organization to assess the efficiency and effectiveness of its processes. By analyzing performance indicators such as submission turnaround times, approval rates, and regulatory milestones achieved, organizations can identify areas for improvement.
How does this optimize your system?
Analytics and reporting tools help organizations monitor the health of their processes, find bottlenecks, and have real-time insights into data and trends, advancing performance. This streamlines the product lifecycle and reduces the time-to-market period.
4. Go Beyond the Auto-On Features
Veeva rolls out general updates every four months with a mix of “auto-on” and configurable features across all applications. While auto-on, or auto-enabled updates, are convenient to the update process, they may overlook potentially important items. This is especially pertinent for Vault RIM, where regulatory information could be impacted.
Data migration experts recommend a thorough review of Network Release Notes to ensure no beneficial features are missed. Veeva release notes are intuitively structured and articulate, so there is no exhaustive overhead. In fact, release notes foster communication between users, as there may be circumstances where individual input on update features is required.
How does this optimize your system?
Reading through release notes ensures that applicable features are understood and enabled. This practice allows organizations to leverage the full potential of the system, address any potential issues or concerns, and tailor the system to meet specific user needs and preferences.
5. Use Vault Connections
Vault Connections are Veeva-delivered integrations that seamlessly transfer data and documents between applications. They are designed to streamline cross-functional business processes by providing greater visibility and automating manual tasks.
How does this optimize your system?
Built-in Connections allow for a flow of information in and out of the Vault system. This not only simplifies your data hand-offs but it also automates your processes.
Connectors make sure all departments have access to relevant data in real-time, improving efficiency across your organization’s software.
What Happens After Optimization?
While optimization is a great first step toward streamlining the product cycle, it’s important to recognize a system can never be perfectly optimized.
Periodic reviews look for growth opportunities and encourage communication among Vault users. It’s important to continue this engagement with administrators and project stakeholders, as input from all levels of an organization ensures the configuration meets expectations across all functional areas.
Conclusion
Although Veeva Vault is a crucial regulatory asset, its optimization boosts workflow effectiveness. Consider partnering with optimization experts who offer regulatory and system expertise. These partners delve into your system, identifying communication gaps and implementing the best configuration practices for your specific needs.
A great first step towards enhanced performance is a ‘Health Check’ with the Veeva Vault RIM questionnaire. The questionnaire is a system and process review of your organization’s Vault RIM configuration and usage. This process evaluates your business needs and ensures your system is performing at optimal levels.
Daelight Solutions is a certified Veeva Vault Migration Partner, helping businesses migrate, implement, manage, and optimize Veeva Vault services. Contact us today to discuss how to take your Veeva Vault RIM to the next level.
